The research poster entitled, Demographics of Professors Teaching Industry-Related Courses within Accredited United States Colleges of Pharmacy, builds upon previous IPhO research published last year describing trends among industry-related courses within US accredited colleges of pharmacy.
Authors: Andy Szeto (pictured at left), University of the Pacific; Tayyeb Din, Long Island University; Marine Schmitt, Oregon State University; Rosemary Boshar, PharmD, Sanofi Genzyme/MCPHS Fellow (pictured at right); and Jerry Silverman, BS Pharm, Industry Pharmacists Organization, describe professor experience among industry-related courses within US accredited colleges of pharmacy.
This research shows a large variation in instructor experience ranging from 1 year to 40 years, with a mode of just one year of industry pharmacy experience. The authors hope that as student interest in industry continues to escalate, ACPE will revise their curriculum requirements to include industry topics, and in time this will facilitate a need for full-time industry-experienced professors.
To review this research poster, please click here to visit the IPhO Scholarly Publications section of our website.
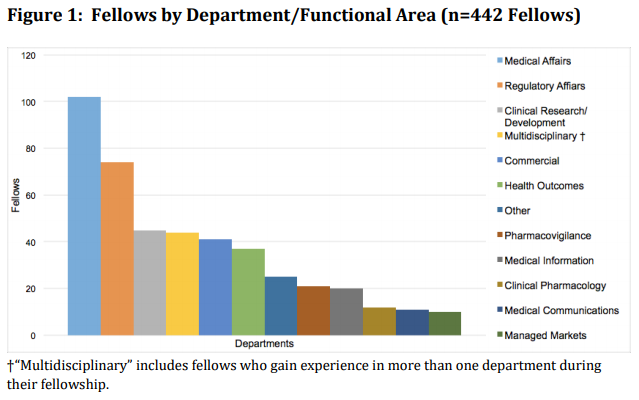
The second research poster presented at Midyear 2017, Demographics of Current First- and Second-year Industry Fellows, reports upon changes in current fellows’ academic and experiential backgrounds to better prepare and guide applicants toward obtaining an industry fellowship.
 Congratulations to student pharmacists at Pacific University School of Pharmacy. IPhO now connects them to a national network of over 4,000 student pharmacists with similar interests, as well as a national network of hundreds of industry fellows and thousands of industry pharmacists.
Congratulations to student pharmacists at Pacific University School of Pharmacy. IPhO now connects them to a national network of over 4,000 student pharmacists with similar interests, as well as a national network of hundreds of industry fellows and thousands of industry pharmacists.











.jpg)
